Des chercheurs des universités Paris Cité & Sorbonne, de l’INSERM, du CNRS, et de l’AP-HP ont publié cette semaine un article dans la revue Science Translational Medicine démontrant que la dérégulation de la flore intestinale conduit au développement d’une maladie auto-immune. Les travaux ont été réalisés principalement par le Dr James Gleeson dans le Centre de Recherche sur l’Inflammation à la faculté de médecine du site de l’hôpital Bichat au sein de l’équipe du Pr Monteiro et au Centre de Recherche de l’hôpital Saint-Antoine dans l’équipe du Pr Sokol.
L’analyse des microbiotes chez les patients atteint d’une maladie autoimmune a révélé une dysbiose sous la forme d’un excès d’une bactérie commensale, Akkermansia muciniphila. Jusqu’à présent, cette bactérie est considérée comme protectrice des troubles métaboliques (obésité, diabète); à cet effet, elle est commercialisée en ligne.
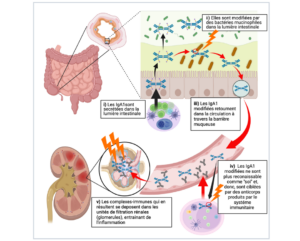
Les chercheurs se sont penchés sur une maladie auto-immune qui touche les reins, appelée néphropathie à dépôts d’immunoglobuline A (NIgA), ou Maladie de Berger, découverte en France par le Pr Jean Berger en 1968. Chaque année, cette maladie est diagnostiquée en France chez environ 3 individus sur 100.000, le plus souvent des hommes entre 20 à 40 ans; elle est une cause fréquente d’insuffisance rénale dans le monde, pouvant nécessiter le recours à la dialyse ou à la greffe de rein.
muciniphila digère des chaînes de sucre pour sa croissance. Ces sucres sont très représentés dans l’IgA1, une glycoprotéine humaine, ce qui en fait une cible de ces bactéries. Les résultats montrent que ces bactéries peuvent modifier les glycanes liés à la région charnière des IgA humaine de type 1 (IgA1) après leur sécrétion dans la lumière intestinale et générer ainsi des déterminants antigéniques nouveaux; après le passage dans le sang de ces IgA1 dé-glycosylées, ces déterminants antigéniques deviennent donc la cible du système immunitaire du patient. Ce processus initie la formation de complexes immuns à l’origine des lésions glomérulaires rénales. Chez les souris, l’enrichissement du microbiote en A. muciniphila aggrave la maladie rénale tandis que la déplétion du microbiote intestinal par des antibiotiques l’atténue.
Ces résultats améliorent notre compréhension du rôle de la flore intestinale dans les maladies auto-immunes ; ils incitent à la prudence dans les manipulations de la flore intestinale. Ils mettent en lumière le rôle de la flore intestinale comme un facteur environnemental susceptible d’initier une maladie auto-immune et identifie des nouvelles cibles thérapeutiques dans la perspective d’un traitement pour la néphropathie à dépôts d’IgA.
.png)