Abstract
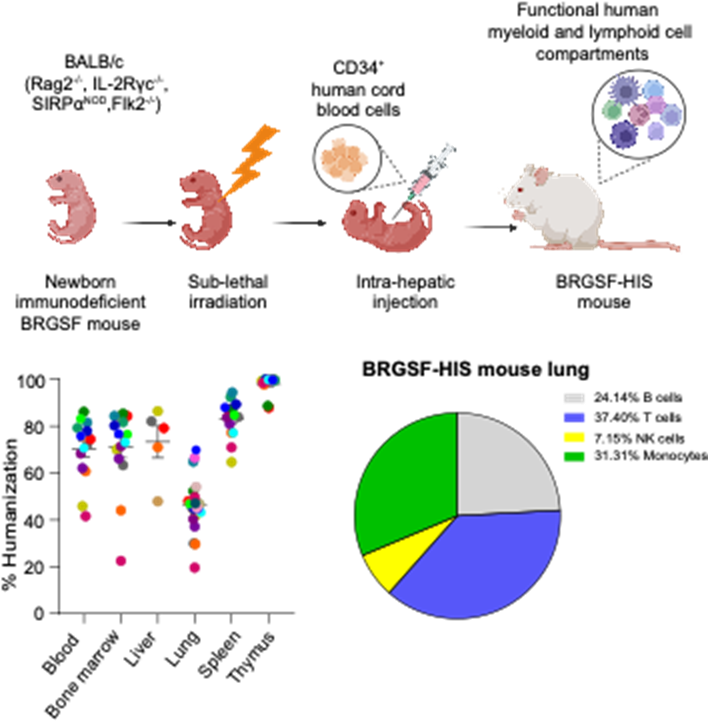
Humanized mice represent robust models for the study of small-molecule drugs, antibodies, and gene therapies. Indeed, compared to classical murine models, they more accurately reflect human diseases and responses. A humanized mouse model built on the BRGSF (BALB/c Rag2−/− IL-2Rγc−/− SIRPαNOD Flk2−/− Flt3tm1lrl) genetic background was recently established. Following irradiation, it can be successfully reconstituted with human blood-cord-derived CD34+ hematopoietic stem cells to establish a fully functional human immune system (HIS). Lung immunology is emerging as an area of increasing importance, particularly in light of the role of the immune system in the pathology of COVID-19 and of the growing burden of respiratory diseases as a result of industrialization and increased exposure to pollutants and allergens. In this article, we characterized the immunological landscape of the lungs in BRGSF-HIS mice. In the alveolar spaces, only macrophages of mouse origin were detected, while in the lung interstitium, human natural killer cells and various T cell subsets were present, including CD4+ and CD8+ T cells, γδ T cells, and regulatory T cells, with percentages comparable to those in human lungs. Human CD14+ monocytes and dendritic cells of BRGSF-HIS mice were predominant compared to other myeloid populations, while neutrophils were underrepresented. However, treatment of mice with exogenous human granulocyte colony-stimulating factor increased the pool of human neutrophils. Adherent myeloid cells from BRGSF-HIS mouse lungs were responsive to LPS stimulation, which led to human cytokine secretion, confirming the ability of myeloid cells to mount inflammatory responses. These findings indicate that BRGSF-HIS mice exhibit human myeloid and lymphoid cell compartments within the lung. While certain limitations must be considered when determining their suitability for specific research applications, BRGSF-HIS mice offer valuable insight into immune mechanisms relevant to select human lung diseases.